GLOBAL HARMONIZATION WORKING PARTY (GHWP)
28th GHWP ANNUAL MEETING AND 28th GHWP TC MEETING
About GHWP
Global Harmonization Working Party (GHWP) is a non-profit organization involving the participation of medical device regulatory authorities and industry representatives across the globe. Its predecessor was the Asian Harmonization Working Party (AHWP) predominated by Asian countries and regions. With the growing number of members and increasing global impact, AHWP had transformed from an Asian organization into an international organization. In 2021, it was renamed as the GHWP, with the aim to promote global medical device regulation toward greater convergence, harmonization and reliance. It has now expanded its membership to 34 countries and regions, from Asia to the North and South Americas as well as Africa. To work in collaboration with related international organizations such as IMDRF, WHO, ISO, and IEC. Through the dialogue and communication between medical device regulatory authorities and the industry, it works towards the continuous promotion of high-quality development in global medical device regulation and industry.
About GHWP
Global Harmonization Working Party (GHWP) is a non-profit organization involving the participation of medical device regulatory authorities and industry representatives across the globe. Its predecessor was the Asian Harmonization Working Party (AHWP) predominated by Asian countries and regions. With the growing number of members and increasing global impact, AHWP had transformed from an Asian organization into an international organization. In 2021, it was renamed as the GHWP, with the aim to promote global medical device regulation toward greater convergence, harmonization and reliance. It has now expanded its membership to 34 countries and regions, from Asia to the North and South Americas as well as Africa. To work in collaboration with related international organizations such as IMDRF, WHO, ISO, and IEC. Through the dialogue and communication between medical device regulatory authorities and the industry, it works towards the continuous promotion of high-quality development in global medical device regulation and industry.
Event Highlights

In conjunction with International Medical Device Exhibition & Conference (IMDEC) 2024 from 10 - 12 December 2024

Closing Ceremony, presided over by
YAB Dato' Seri Anwar Ibrahim,
Prime Minister of Malaysia

Inaugural ceremony by
YB Datuk Seri Dr. Dzulkefly Ahmad, Minister of Health, Malaysia. Let's shape the future of medical device regulation together!

Stay up-to-date with the most recent insights and updates, where regulatory excellence and industry expertise are at the forefront
Stay up-to-date with the most recent insights and updates, where regulatory excellence and industry expertise are at the forefront
Capacity Building Topics for 2024:
- Global Regulatory Partners (GRP) & Good Submission Practice
- Clinical Evidence & Real-world Evidence
- Cybersecurity in Medical Device
- Innovative Medical Devices Approval Pathway
- Orphan Medical Device Challenges
- Post-Market Surveillance (PMS) for Medical Devices
- Risk Management in Innovative Devices
- Regulatory Convergence & Reliance
*Please note that the above programs may be subject to change
EVENT HIGHLIGHTS

In conjunction with International Medical Device Exhibition & Conference (IMDEC) 2024 from 10 - 12 December 2024

Closing Ceremony, presided over by
YAB Dato' Seri Anwar Ibrahim,
Prime Minister of Malaysia

Inaugural ceremony by
YB Datuk Seri Dr. Dzulkefly Ahmad, Minister of Health, Malaysia. Let's shape the future of medical device regulation together!

Stay up-to-date with the most recent insights and updates, where regulatory excellence and industry expertise are at the forefront
Stay up-to-date with the most recent insights and updates, where regulatory excellence and industry expertise are at the forefront
Capacity Building Topics for 2024:
- Global Regulatory Partners (GRP) & Good Submission Practice
- Clinical Evidence & Real-world Evidence
- Cybersecurity in Medical Device
- Innovative Medical Devices Approval Pathway
- Orphan Medical Device Challenges
- Post-Market Surveillance (PMS) for Medical Devices
- Risk Management in Innovative Devices
- Regulatory Convergence & Reliance
*Please note that the above programs may be subject to change
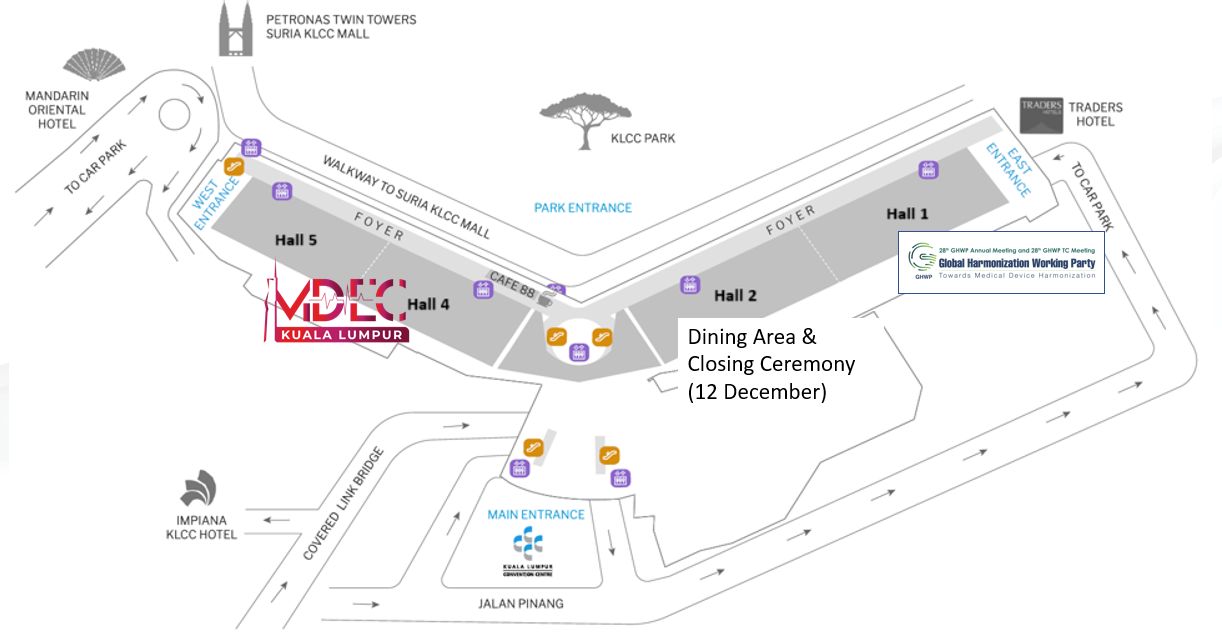
VENUE : KLCC Convention Centre, Kuala Lumpur, Malaysia
About The Venue
KLCC Convention Centre The Kuala Lumpur Convention Centre, also known as the KL Convention Centre, is a convention and exhibition centre located in the Kuala Lumpur City Centre development in Kuala Lumpur, Malaysia.
VENUE : KLCC Convention Centre, Kuala Lumpur, Malaysia

About The Venue
KLCC Convention Centre The Kuala Lumpur Convention Centre, also known as the KL Convention Centre, is a convention and exhibition centre located in the Kuala Lumpur City Centre development in Kuala Lumpur, Malaysia.
Supported by


Supported by


























































































































































































































































































































